Causality assessment of AEFIs
You can read more on causality assessment in the Global Advisory Committee on Vaccine Safety (GACVS)![]() Global Advisory Committee on Vaccine Safety (GACVS)Established in 1999, the GACVS advises the WHO on vaccine-related safety issues and enables WHO to respond promptly, efficiently, and with scientific rigor to issues of vaccine safety with potential global importance. The committee also assesses the implications of vaccine safety for practice worldwide and for WHO policies. report "Causality assessment of adverse events following immunization" that includes other conditions and provisions that should be applied in evaluating causality in the field of vaccine safety.
Global Advisory Committee on Vaccine Safety (GACVS)Established in 1999, the GACVS advises the WHO on vaccine-related safety issues and enables WHO to respond promptly, efficiently, and with scientific rigor to issues of vaccine safety with potential global importance. The committee also assesses the implications of vaccine safety for practice worldwide and for WHO policies. report "Causality assessment of adverse events following immunization" that includes other conditions and provisions that should be applied in evaluating causality in the field of vaccine safety.
Most countries have AEFI systems and attach great importance to reports of suspected adverse events. These systems have been successful in identifying severe AEFIs after vaccines are licensed. Follow-up studies are usually needed to further investigate causality of AEFIs.
Although the most reliable way to determine whether an adverse event is causally related to vaccination is through a randomized clinical trial, such trials are limited to the clinical development phase of vaccines. Once a vaccine is licensed, controlled trials are no longer an option owing to ethical reasons (withholding vaccination).
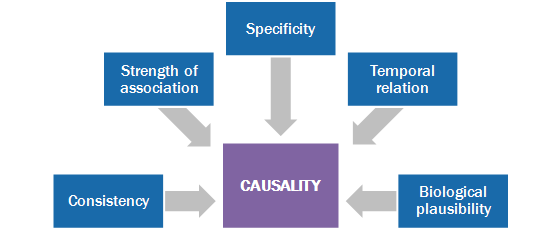
Causality assessment is the systematic review of data about an AEFI case. It determines the likelihood of a causal association between the event and the vaccine(s) received. Causality assessment helps determine:
- If an AEFI is attributable to the vaccine or the vaccination programme,
- What steps – if any – need to be taken to address the event.
The WHO Aide-Memoire on causality assessment serves as a guide to a systematic, standardized causality assessment process for serious adverse events following immunization (including clusters).36
WHO Aide-Memoire: AEFI: Causality AssessmentCausality assessment outcomes help raise awareness of vaccine associated risks among health-care workers. This, combined with knowledge of benefits of immunization, forms the basis of vaccine information for parents and/or vaccinees.
The quality of a causality assessment depends on:
- Quality of AEFI case report,
- Effectiveness of AEFI reporting system,
- Quality of the causality review process.
There are five principles that underpin the causality assessment of vaccine adverse events.35